Sterilizing existing mix in container
crystals1943
10 years ago
Related Stories

BATHROOM DESIGNHow to Mix Metal Finishes in the Bathroom
Make a clean break with one-dimensional bathroom finishes by pairing nickel, silver and bronze hardware and fixtures
Full Story
GARDENING GUIDESVegetables and Flowers Mix in Beautiful Edible Gardens
Ornamentals, meet your edible garden mates. We know you'll get along just beautifully
Full Story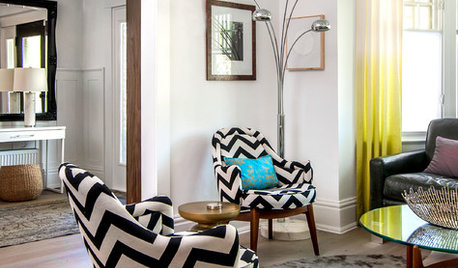
HOUZZ TOURSHouzz Tour: Mixing It Up in a Century-Old Edwardian
Different eras, patterns and textures mingle beautifully in a Canadian interior designer's home and 'design lab'
Full Story
ECLECTIC HOMESHouzz Tour: High-Low Mix in a Colorful Victorian
An unloved house is transformed into a cheerful, versatile home with a blend of design classics, budget pieces and treasured finds
Full Story
HOUZZ TOURSMy Houzz: Mixed-Use Oregon Home Serves and Charms
Home, restaurant, garden, rental cabin — and it gives back to the community too. This multitasking home is a wonder in more ways than one
Full Story
LANDSCAPE DESIGNGet More From Your Garden by Mixing Things Up
Consider an eclectic outdoor style with defined hardscapes softened by exuberant, informal plantings
Full Story
KITCHEN DESIGNNew and Old Mix It Up in a Historic Farmhouse Kitchen
A couple rethink the kitchen in their Pennsylvania farmhouse to restore authenticity while also creating a space for modern living
Full Story
CONTAINER GARDENS7 Deer-Resistant Flowers for Your Summer Containers
Grow these as protection for edibles or just for their colorful beauty — deer might not like them, but everyone else will
Full Story
CONTAINER GARDENS9 Tips for Creating an Artful Container Garden
Make your potted plantings a beautiful sight with these ideas for container types, plant groupings and more
Full StoryMore Discussions










nil13
oxboy555
Related Professionals
Belvedere Park Landscape Contractors · Columbine Landscape Contractors · Dedham Landscape Contractors · Forest Hills Landscape Contractors · Hayden Landscape Contractors · Parker Landscape Contractors · Stallings Landscape Contractors · Yuba City Landscape Contractors · San Francisco Window Contractors · Minnetonka Window Contractors · Sayville Window Contractors · Sebring Window Contractors · South Laurel Window Contractors · Annapolis Fence Contractors · Yorba Linda Fence Contractorsweirdflowers