Lime with partially acidic plants?
MiniGerdener876
11 years ago
Related Stories

GARDENING GUIDESGreat Design Plant: Eastern Bluestar Wows in Fall
Get outstanding late-season color with this clumping perennial, perfect for a mixed border or to partially replace turf
Full Story
GARDENING GUIDESGreat Design Plant: Juniperus Conferta ‘Golden Pacific’
‘Golden Pacific’ shore juniper shines in sun or partial shade
Full Story
GARDENING GUIDESGreat Garden Combo: 6 Beautiful Plants for a Shady, Wet Site
Transform a shade garden with moisture-loving golden grasses, textural leaves and a sprinkling of flowers
Full Story
FOLIAGEGreat Design Plant: Ornamental Sweet Potato Vine
Versatile, fast growing, inexpensive and easy on the eyes, ornamental sweet potato vine has it all
Full Story
LIME FOLIAGE5 Gold Plants to Illuminate Shady Garden Spots
Dark garden corners don't have to mean deep, monochromatic color. Gold plants brighten the landscape with shots of luminosity
Full Story
GARDENING AND LANDSCAPINGGreat Design Plant: Golden Creeping Jenny
Try this fast-growing ground cover for easy masses of gold and green in the garden
Full Story
LIME FOLIAGEGreat Design Plant: Illicium Parviflorum ‘Florida Sunshine’
This shrub from the anise family brings a ray of chartreuse sunlight to the woodland garden
Full Story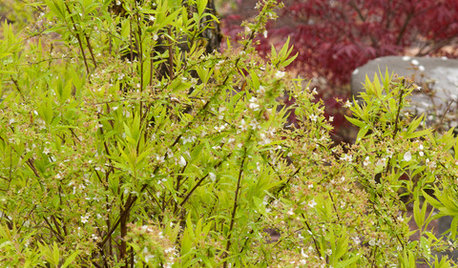
GARDENING GUIDESGreat Design Plant: Ogon Spirea for Radiance and Texture
This feathery shrub will light up your garden with its bright color and easygoing personality
Full Story0

TREESGreat Design Plant: Coral Bark Japanese Maple, a Winter Standout
Go for garden gusto during the chilly season with the fiery red stems of this unusual Japanese maple
Full Story
FOLIAGEGreat Design Plant: Foxtail Fern
Not actually a fern, this vivid member of the asparagus family has a distinctive appearance to awaken a garden year-round
Full StoryMore Discussions







Ernie
MiniGerdener876Original Author
Related Professionals
Windham Landscape Architects & Landscape Designers · Panama City Landscape Architects & Landscape Designers · Southfield Landscape Architects & Landscape Designers · Framingham Landscape Contractors · Hayward Landscape Contractors · Hicksville Landscape Contractors · Lemoore Landscape Contractors · Riverhead Landscape Contractors · South Hackensack Landscape Contractors · Goldenrod Landscape Contractors · Glendale Heights Window Contractors · Scotts Valley Window Contractors · North Miami Beach Fence Contractors · Sandy Springs Fence Contractors · Centreville Fence Contractorsgreenman28 NorCal 7b/8a
DWD2
Ernie
greenman28 NorCal 7b/8a
MiniGerdener876Original Author
DWD2
DWD2