How Much Elemental Sulphur To Apply?
Ghadames
9 years ago
Related Stories

BATHROOM DESIGN7 Elements of Craftsman-Style Bathrooms
If you like a handmade look and simple materials, this style is tailor made for your bath
Full Story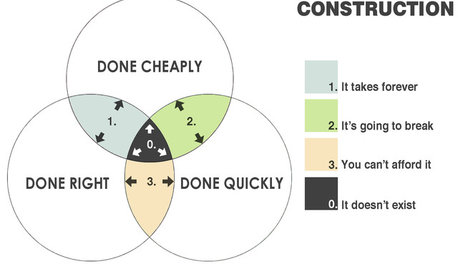
COFFEE WITH AN ARCHITECTThe Elements of Design Explained With Venn Diagrams
Design doesn't have to be hard to understand. It just needs the right presentation
Full Story
BATHROOM STYLES8 Elements of Beach-Style Bathrooms
Use seaside colors, raw finishes and coastal accessories to conjure the perfect outing in your indoor bath
Full Story
9 Elements of Asian Style
Let Your Bath Be Inspired by an Appreciation of Nature, Simplicity and Asymmetry
Full Story
ENTRYWAYSGrand Entry Elements: Newel Posts Past and Present
They once spoke to wealth and class, but newel posts today say more about individual style
Full Story
CRAFTSMAN DESIGNAmerican Architecture: The Elements of Craftsman Style
Proud of its handiwork details and with nature as inspiration, Craftsman architecture stands out for its purity of style
Full Story
DISASTER PREP & RECOVERY5 Essential Elements of a Storm Evacuation Plan
Be ready to make a quick getaway from a storm with these tips for packing up, planning and protecting your home while you're away
Full Story
BATHROOM DESIGN6 Elements of a Perfect Bathroom Paint Job
High-quality paint alone won't cut it. For the best-looking painted bathroom walls, you'll need to get these other details right
Full Story
KITCHEN WORKBOOK15 Elements of a Traditional Kitchen
Small details take center stage with decorative moldings, glazed finishes, raised panels and more
Full Story
BATHROOM WORKBOOKBathroom Workbook: 6 Elements of Eclectic Style
Express your unique self by mixing styles, eras and more, for a bathroom that’s full of personality
Full Story









charina
MrClint
Related Professionals
Beachwood Landscape Architects & Landscape Designers · Fort Lee Landscape Architects & Landscape Designers · Mitchellville Landscape Architects & Landscape Designers · Towson Landscape Architects & Landscape Designers · Burlington Landscape Contractors · Clermont Landscape Contractors · Matthews Landscape Contractors · Byram Landscape Contractors · Fort Hunt Landscape Contractors · Mercedes Landscape Contractors · Overland Park Landscape Contractors · West Orange Landscape Contractors · New Carrollton Landscape Contractors · Winter Gardens Landscape Contractors · Raytown Landscape Contractorsjtburton
alan haigh