Science lesson needed
hardin
14 years ago
Related Stories

ROOM OF THE DAYRoom of the Day: Art and Science Room Proves Grandmas Are the Best
This grandmother transformed her garage into a science and art classroom where messes are encouraged every day
Full Story
EVENTSOn Show: Weird, Wondrous Science Meets Design
Houses grown, not built. Power-generating soil. And snail poop that ... well, see for yourself in our coverage of a new Rotterdam exhibit
Full Story
HOUZZ TOURSDesign Lessons From a 10-Foot-Wide Row House
How to make a very narrow home open, bright and comfortable? Go vertical, focus on storage, work your materials and embrace modern design
Full Story
GARDENING GUIDES5 Invaluable Life Lessons From the Garden
The garden is both teacher and healer. Don't be afraid — dig in and reap the benefits
Full Story
PRODUCT PICKSGuest Picks: Mad Science
Get inspired by science fiction with 20 decorative items pulled straight from your favorite B movie
Full Story
PRODUCT PICKSGuest Picks: Science-Inspired Design Finds
Look to geology, chemistry, geography and other sciences for inspiration in home decor
Full Story
DECORATING GUIDES16 Lounges Go Wild for Science at San Francisco's Exploratorium
See the imaginative designs concocted and let us know which style formula works best for you
Full Story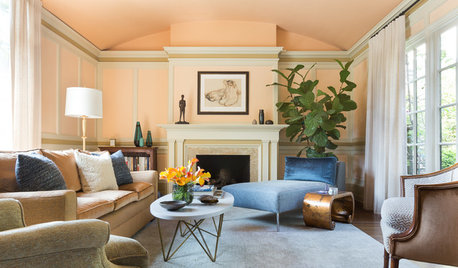
TRANSITIONAL HOMESHouzz Tour: The Science of Blending Old and New
An interior designer helps clients pull together modern furniture and heirloom pieces in a classic home
Full Story
SELLING YOUR HOUSEA Moving Diary: Lessons From Selling My Home
After 79 days of home cleaning, staging and — at last — selling, a mom comes away with a top must-do for her next abode
Full StoryMore Discussions








horton
sleeplessinftwayne
Related Professionals
Danbury Landscape Architects & Landscape Designers · Clemson Landscape Architects & Landscape Designers · Anderson Landscape Contractors · Chattanooga Landscape Contractors · East Lake-Orient Park Landscape Contractors · El Segundo Landscape Contractors · Fairfield Landscape Contractors · Fort Wayne Landscape Contractors · La Vista Landscape Contractors · New Cassel Landscape Contractors · Post Falls Landscape Contractors · Seminole Landscape Contractors · Vashon Landscape Contractors · Watertown Landscape Contractors · Whittier Landscape Contractorssleeplessinftwayne
hardinOriginal Author
drh1
squirelette
hardinOriginal Author
drh1
pondbucket
hardinOriginal Author
horton
drh1
hardinOriginal Author