soil ph and water ph are high???
pepper71
13 years ago
Related Stories

GARDENING GUIDESGrow a Beautiful Garden in Alkaline Soil
Got alkaline soil? Learn how to manage it and the many beautiful plants that will thrive in this ‘sweet’ soil
Full Story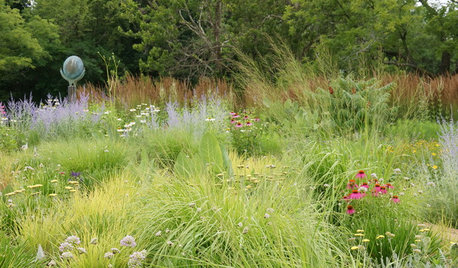
GARDENING GUIDESGet the Dirt on Your Garden’s Soil
Understand how your soil supports your plants so you can ensure your garden’s success
Full Story
GARDENING GUIDESHow to Stop Worrying and Start Loving Clay Soil
Clay has many more benefits than you might imagine
Full Story
FARM YOUR YARDHow to Get Good Soil for Your Edible Garden
The nutrients in your soil feed the plants that feed you. Here are tips on getting it right — just in time for planting season
Full Story
GARDENING GUIDESGardening Solutions for Heavy Clay Soils
What’s a gardener to do with soil that’s easily compacted and has poor drainage? Find out here
Full Story
CONTAINER GARDENSContainer Gardening Basics: The Dirt on Soil
Learn the types of potting soil available and the best mixes to help your containers thrive
Full Story
PRODUCT PICKSGuest Picks: High-Tech Plant Helpers
Hydroponics, monitoring systems, even an electric pollinator ... these gadgets and services keep your greenery growing strong
Full Story
LIGHTINGThe Lowdown on High-Efficiency LED Lighting
Learn about LED tapes, ropes, pucks and more to create a flexible and energy-efficient lighting design that looks great
Full Story
LANDSCAPE DESIGNSecrets of a Successful Water Garden
Relax. Having a water garden is much easier once you understand the basics
Full Story
GARDENING GUIDESGreat Design Plant: Wild Lupine Dresses Up Rocky Gardens
Spiky blue flowers and a high tolerance for poor soil make this plant ideal for tough sites
Full Story





ericwi
Michael
Related Professionals
Benbrook Landscape Architects & Landscape Designers · Lake Oswego Landscape Architects & Landscape Designers · Waunakee Landscape Architects & Landscape Designers · Cudahy Landscape Contractors · Manhattan Landscape Contractors · North Plainfield Landscape Contractors · Olympia Landscape Contractors · Severna Park Landscape Contractors · Tyngsboro Landscape Contractors · San Pablo Landscape Contractors · Alvin Decks, Patios & Outdoor Enclosures · Bloomington Decks, Patios & Outdoor Enclosures · Champaign Decks, Patios & Outdoor Enclosures · Kissimmee Decks, Patios & Outdoor Enclosures · Oswego Decks, Patios & Outdoor EnclosuresKimmsr
ceth_k
gargwarb